Self - Supplying Oxygen Bionic Nanomaterials Combat Drug - Resistant Bacteria
In a significant stride towards combating the global threat of drug - resistant bacteria, researchers have developed a novel bionic nanomaterial that can self - supply oxygen, offering a potential breakthrough in treating stubborn bacterial infections.
Drug - resistant bacteria, also known as "superbugs," have become a major concern in modern medicine. The overuse and misuse of antibiotics over the years have led to the evolution of bacteria that can withstand the effects of these drugs. This has made common infections, such as those caused by methicillin - resistant Staphylococcus aureus (MRSA) and multidrug - resistant Escherichia coli, much more difficult to treat.
The Innovation of Self - Supplying Oxygen Nanomaterials
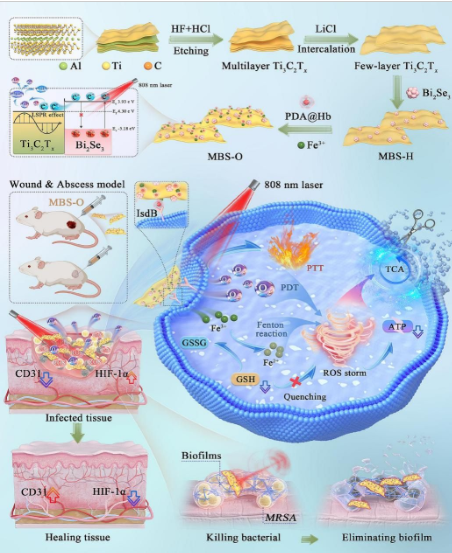
The newly developed bionic nanomaterials are designed to mimic the oxygen - releasing mechanisms found in natural biological systems. These nanomaterials are composed of a core - shell structure, with the core containing oxygen - generating substances and the shell being made of a biocompatible material that can target bacterial cells.
When the nanomaterials are introduced into an environment with bacteria, they respond to the specific chemical signals or physical conditions in the bacterial micro - environment. For example, they can detect the low - oxygen levels typically associated with the presence of bacteria. In response, the oxygen - generating core of the nanomaterials starts to release oxygen, creating a more oxygen - rich environment around the bacteria.
How It Works Against Drug - Resistant Bacteria
Oxygen is crucial for the antibacterial activity of many natural and synthetic substances. In the case of these self - supplying oxygen nanomaterials, the released oxygen has multiple effects on drug - resistant bacteria.
Firstly, the increased oxygen concentration can enhance the activity of certain antibacterial agents that rely on oxygen - related chemical reactions to work. Some antibacterial substances can react with oxygen to form highly reactive oxygen species (ROS), such as hydroxyl radicals and superoxide anions. These ROS are extremely effective in damaging the cell membranes, DNA, and proteins of bacteria, leading to their death. Even drug - resistant bacteria are vulnerable to the oxidative stress caused by these ROS.
Secondly, the oxygen - rich environment created by the nanomaterials can disrupt the normal metabolic processes of bacteria. Many drug - resistant bacteria have adapted to survive in low - oxygen conditions, and the sudden influx of oxygen can overwhelm their metabolic systems. This disruption can lead to a halt in the bacteria's growth and reproduction, effectively neutralizing their ability to cause infection.
Promising Experimental Results
Initial laboratory experiments have shown remarkable results. In tests against MRSA, one of the most common and dangerous drug - resistant bacteria, the self - supplying oxygen nanomaterials, in combination with a commonly used antibacterial agent, were able to reduce the bacterial population by over 90% within a few hours. This is a significant improvement compared to the use of the antibacterial agent alone, which had only a marginal effect on the drug - resistant MRSA.
Similar positive results were also observed in experiments with multidrug - resistant Escherichia coli. The nanomaterials not only enhanced the antibacterial activity but also showed the potential to prevent the formation of bacterial biofilms. Biofilms are communities of bacteria that are encased in a protective matrix, making them even more resistant to antibiotics and the body's immune system. By disrupting the early stages of biofilm formation, the self - supplying oxygen nanomaterials could potentially prevent the development of chronic and difficult - to - treat infections.
Future Applications and Challenges
The development of these self - supplying oxygen bionic nanomaterials opens up new possibilities for the treatment of drug - resistant bacterial infections. In the future, they could be used in a variety of medical applications, such as in wound dressings to prevent or treat infected wounds, in catheters to reduce the risk of catheter - related infections, and in the treatment of internal infections where traditional antibiotics have failed.
However, there are still challenges to overcome before these nanomaterials can be widely used in clinical settings. One of the main challenges is ensuring their safety and biocompatibility in the human body. Although initial tests have shown that the nanomaterials are well - tolerated by cells in the laboratory, more extensive in - vivo studies are needed to confirm their safety in animals and humans. Additionally, optimizing the manufacturing process to produce these nanomaterials on a large scale and at a reasonable cost will also be crucial for their widespread adoption.